Cold Light: Luminescence
The html formatting and custom instructions have been disabled on this server. The same file with adapted formatting can be found here: https://farbeinf.de/static_html/luminescence.html.
Transitions between energy levels of molecules, atoms, or any other system can always occur in both directions: radiation which can be absorbed, can also be emitted. But as molecules can give off their excitation energy easily by interaction with their neighbours – transforming it into thermal motion – absorption largely outweighs emission and the latter is not observed. There are, however, exceptions:
if, in the dark, energy is transmitted to a molecule (or crystal etc.) by a mechanism other than light, then emission can be seen. This is rather rare in nature – fireflies, glowworms, some deep-sea animals, some micro-organisms in the sea.
The energy can be supplied by ultraviolet irradiation, by X-rays, electron beam impact, by electric fields, chemical reactions or mechanical stress.
Light emission from atoms, e.g. in electric gas discharges, may also be considered to belong to luminescence phenomena. Because of its significance for the knowledge of atomic structure, this has been dealt with in a separate section. Here larger molecules and solids are considered which may be excited to luminesce with comparatively small energies.
If the energy transmitted by irradiation is sufficiently large, even water is shining, as seen on the picture to the right which shows the core of a research nuclear reactor (TRIGA Mark 3) using water as moderator. |
 |
BioluminescenceBioluminescence is a rather rare sight. Glowworms or fireflies, microorganisms in the sea (marine phosphorescence), many deep-sea animals, and some fungi show it.
To the right: the glowworm Lampyris noctiluca. |
 |
Friction
Luminescence due to mechanical stress, friction in particular, is called triboluminescence. On the scale of typical molecular excitation energies, the energy transferred is huge. Chemical bonds are broken and electric charges are separated. Correspondingly, there is a variety of consecutive processes which may lead to the emission of light. Practical uses of triboluminescence in technology are scarce, and therefore there is only little research in this field.
An easily available substance which shows this effect is sugar. Grinding sugar or rubbing two pieces of lump sugar on each other when it is dark, one can see the glow, if the eyes are well adapted to darkness (it must be pitch-dark). However, the glow appears white, as colour vision needs higher intensities.
The emission is mainly in the blue and ultraviolet region. The spectrum has been analyzed by
Zink et al. (J. Phys. Chem., 1976, 80 (3), pp 248–249) and has been identified as emission spectrum of molecular nitrogen N
2.
After degassing the sugar by moderate heating in the vacuum, in nitrogen-free surrounding triboluminescence could not be observed any more.
Triboluminescence of quartz is much brighter. Quartz pebbles are abundant. The orange coloured, spark-like light can be seen in the dark, when two pebbles are rubbed on each other with pressure. There are really no sparks: under water the effect is the same.


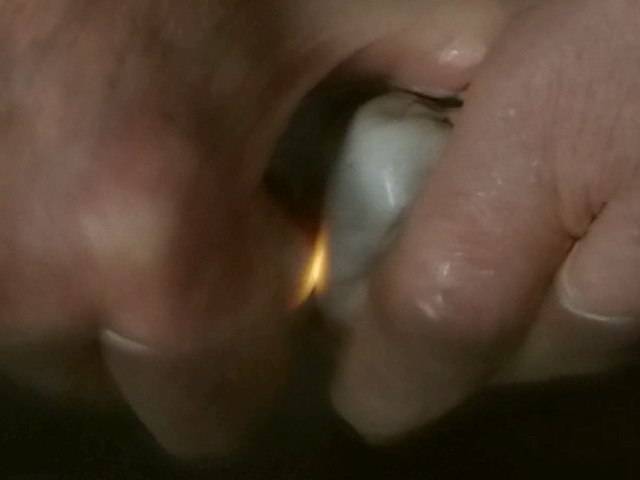
Left: Triboluminescence of quartz pebbles can be seen even in dimmed daylight.
Middle: If the pebbles are transparent the light illuminates the whole stones from inside.
Right: Under water.
According to
Chapman & Walton (J. Appl. Phys. 54, 5961 (1983)) the emission spectrum of quartz cut with a diamond-impregnated saw blade resembles that of a black body of 2800 K.
Irradiation
If the excitations decay “immediately”, the phenomenon is called fluorescence, in case of slower decay (seconds, minutes of even longer) one speaks of phosphorescence.
“Black Light”
Sources of near ultraviolet radiation, so-called black-light bulbs or tubes, as used for disco or party illumination, emit long wavelength UV radiation (UVA) peaking at about 366 nm. The pictures below have been produced using a black-light tube.
Examples
Chlorophyll
Chlorophyll fluoresces – but a green leaf held under an UV-lamp looks rather dark, as the excitation energy is used up almost completely by the photosynthetic system. From the website of Dieter Weiss I learned that the common orange lichen or yellow scale Xanthoria parietina shows chlorophyll fluorescence.


Orange lichen on dead twigs of a walnut tree. The chlorophyll contained in the lichen's algae fluoresces red; the bluish-white glow on the bark is supposedly due to bacteria or fungi.
Horse Chestnut
|
Twig of a horse chestnut tree in water, under UVA radiation. The sap contains aesculin, a substance showing blue fluorescence like the brighteners added to most washing agents.
| |  |
Bananas
Ripe bananas also contain an optical brightener, showing blue fluorescence under UVA:

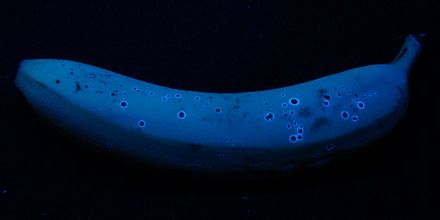
Banana under daylight (left) and under UVA (right).
According to
Bernhard Kräutler et al. (Angewandte Chemie Vol. 120, No. 46 (2008) S. 9087;
"Fluorescent chlorophyll catabolites ...") this is due to a degradation product of chlorophyll.
Swallowwort
Minerals
When dealing with colour of ruby, its fluorescence has already been discussed.
Applications
Fluorescing and also phosphorescing substances are widely used in technology. Fluorescent tubes and lamps contain mercury vapour which in the gas discharge emits visible light, but mainly ultraviolet radiation. The fluorescent coating in the tubes transforms the latter to visible light. Depending on the phosphors of the coating, different colours can be obtained which are well known from advertisement illumination. Cathode ray tubes (as used for television and computer monitors) are examples for fluorescence in vivid colours. The modern flat screens also use phosphors.
The properties of the different phosphors may be found in the manufacturers' web-presentations, e.g. here.
Minerals which themselves are colourless and transparent may become coloured due to impurities, as we have seen. Traces of chromium change colourless corundum to ruby, beryl to emerald. Lattice defects or impurities produce additional energy levels between the energy bands of the solid which make absorption of light possible. Analogously, the additional levels due to defects or impurities can be intermediate steps in the transition of high excitations to the ground state and thus make the emission of light possible. Deliberate addition of foreign atoms is called doping.
Example: Zinc sulphide, ZnS, occurs aa a mineral in two modifications: cubic zinc blende (sphalerite) and hexagonal wurtzite (less frequent). Zinc sulphide is used as a white pigment, mostly in a mixture with barium sulphate. Doped with aluminium, copper, silver or other metals it is a phosphor. ZnS:Ag is used (among others) in cathode ray tubes as blue phosphor. Doping with manganese (ZnS:Mn) yields orange-red fluorescence, doping with copper gives green phosphorescence with long fade-out time. The green luminescence of zinc sulphide has been used errly for X-ray screens, luminous paint, etc.
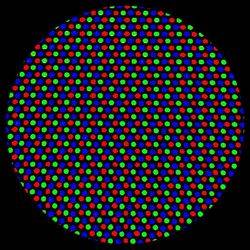 | |
Grey area on a computer screen (CRT), as seen through a strong magnifying glass. The single "dots" are so small that in normal viewing they can not be discriminated. There are only red, green, and blue dots to produce all colours which can be dieplayed. The red phosphor could be Y2O3:Eu (yttrium oxide doped with europium), for the green one ZnS:Cu,Au,Al or Zn2SiO4:Mn could be used and for the blue one, as already mentioned, ZnS:Ag (zinc sulphide doped with silver).
|
Fluorescent Paints and Inks
Fluorescent inks as used for highlighters appear particularly bright because they absorb short wave light and radiate in the medium and long wavelength range. This works particularly well for red to yellow colours.
 | The highlighted area is brighter than the surrounding white paper! The dye (fluorescein or its sodium salt) absorbs short wavelength light (absorption maximum at 494 nm) and gives off a large fraction of it as green fluorescence (maximum at 521 nm) (Data: 1, 2). Together with the remitted light this yields the greenish yellow colour.
|
There are many other uses of fluorescence in chemistry, medicine, geology, and forensics. One example is the use of
fluorescent inks for banknotes as a security measure against counterfeiting:
Link
A good overview over the different types of luminescence is given by Dieter Weiß:
"Lumineszenz" (in German).
Back to the index page "the origins of colour"
Legal Information Data Privacy


