Water
The html formatting and custom instructions have been disabled on this server. The same file with adapted formatting can be found here: https://farbeinf.de/static_html/water.html.
Clean water is crystal-clear, colourless – but in larger bodies of water we see that it is bluish-greenish. The perceived colour of the sea or a lake is a mixture of the colour reflected fron the sky, that of the underground, the water's intrinsic colour, and of suspended particles as algae, cyanobacteria, or mud.

The Baltic Sea.

Glacial stream in the Yoho National Park, British Columbia, Canada. The turbitity due to suspended rock flour brings out and enhances the intrinsic colour of the water. The rock flour itself would look whitish or light grey when dry. (The suspended particles are small, but not small enough to produce colour by the
Tyndall effekt.)
Photo © Henrik Zawischa
 
| A white bucket filled with water (left) or empty (right). The height of the water in the bucket was 25 cm, and the pictures have been taken on a day with overcast sky.
|
In a more expensive experiment, the colour can be seen clearly:
In the picture to thr right, one is looking through a wide acrylic-glass-tube of four meters length filled with clean water against a white wall. (Photograph taken at the Universum® Bremen. The setup can be seen
here.)
|
 |
What is the cause of the faint blue-green colour?
Electronic excitations of the water molecules can not be induced by visible light – the photon energy is too low. UV radiation can induce them. But there are other molecular excitations: vibrations and rotations. To excite rotational modes, tiny amounts of energy are sufficient. The frequencies of vibrations are also much lower than those of light: the absorption maxima for infrared radiation are at wavelengths in the range of 2500 to 3000 nm, far away from the visible region (380 to 760 nm).
In spite of this, vibrations do cause the colour of water. The experimental proof has been given by
C.L. Braun and S.N. Smirnov (J. Chem. Edu., 1993, 70(8), 612) who compared the absorption spectra of water (H2O) and heavy water (D2O).
Let us take a closer look at vibrations.
|
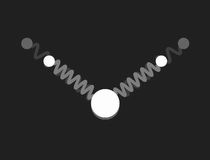
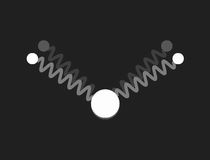
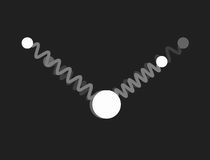
In a free molecule of water – H2O – the angle between the bonds of the two hydrogen atoms to the oxygen is about 104.5°. This causes a large electric dipole moment, as the oxygen carries negative excess charge. The picture to the right shows a mechanical model to visualise the geometry: the bonds are symbolised by massless springs.
|
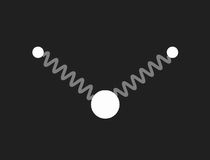 |
Restricting the investigation of the mechanical model to small vibrations, three different modes of harmonic motion and the corresponding frequencies are found. These are shown in the following pictures:
The three normal modes of the mechanical model of H2O.
A double click on an image will start the animation, a single click stops it again. A click on the symbol ◊ opens the animation in a new window.
The vibration frequencies of the three modes have been measured for water vapour (see e.g.
Herzberg, G. Infrared and Raman Spectra; D. Van Nostrand: Princeton, 1945; p. 281); the correspondimng wavelengths of the infrared absorption maxima are 2.74 μm, 7.27 μm, and 2.66 μm, that is far from the visible region which is approximately from 0.4 μm to 0.7 μm.
Harmonic and anharmonic vibrations
Harmonic vibrations result when the restoring force is strictly proportional to the deflection. In most cases this is an idealisation, but nevertheless the harmonic oscillator is one of the most important models in physics.
To keep the discussion simple, we only consider the oscillation of a point particle in a given field of force in one dimension.
If the restoring force is proportional to x (the displacement from the rest position), then the frequency of oscillation is independent of the amplitute. The potential of the force has parabolic shape.
We shall not sketch the quantum mechanical treatment of the harmonic oscillator. Most important is the fact that it can absorb or give off energy only in quanta of hν where h in Planch's constant and ν is the frequency of oscillation. This means that the possible energy levels form a "ladder" of equidistant steps. Moreover, dipole transitions are possible only between adjacent energy levels.
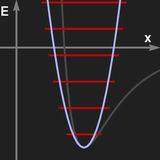
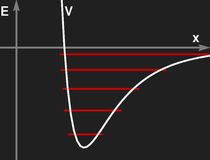
Left: Potential of an harmonic oscillator and the resulting energy levels.
Right: Shape of a realistic effective potential between molecular constituents. In this case, the energy levels are no longer equidistant.
The pictures above show a comparison of a realistic molecular potentian (to the right) and a parabolic potential fitted to yield the same rest position and same frequency for small oscillations.
For the realistic case, even the classical treatment is not quite simple.
Today the easiest method is numerical integration of the equation of motion by means of a computer. This has been done to generate the following image:

Two vibrational solutions with different amplitudes for the realistic potential of the upper right picture.
Small amplitude oscillations are approximately harmonic (= sinusoidal), but for larger amplitude the deviations from the shape of a sinus function become obvious. The curve drawn in white may be approximated as the sum of a constant displacement, a harmonic vibration with the fundamental frequence, and a superposed harmonic vibration with twice the fundamental frequency. (In acoustics such phenomena are well known as overtones which are responsible for the tone colours of musical instruments.)
An oscillator may also resonate if a periodic force with the frequency of its overtones acts on it. This fact has a counterpart in quantum mechanics: whereas "allowed" transitions (= dipole transitions) only occur between adjacent levels of the harmonic oscillator, in anharmonic oscillators they are possible between levels farther apart.
In the water molecule an analogous situation is encountered. The fundamental frequencies are in the infrared, but there is a small probability to excite the higher vibrational levels from the ground state by a single photon. This leads to a little bit of absorption in the long-wave (red) region of the visible spectrum.

Emerald Lake, Yoho National Park, British Columbia, Canada.
© Henrik Zawischa
Water and Ice
The interaction between the molecules in liquid water leads to a slight shift of the absorption maxima and to a broadening of the absorption lines, which become broad humps.
In ice, the hydrogen bonds which hold the molecules on their sites in the lattice couple all the molecules together with the consequence that vibrations propagate as waves through the whole crystal. Of course, these waves are also subject to quantum mechanics; the corresponding quanta are called phonons. Frequency bands result in complete analogy with electron energy bands which have been discussed for
solids. The position of the bands is determined primarily by the vibration frequencies of the free molecules. Thus there are no significant differences in the colours of water and ice.

An iceberg near Spitsbergen. Photo © Philipp Schaudy (site), shown with permission.
Heavy Water
Heavy water does not have this slight bluish tinge of water. Its absorption spectrum is very similar to that of normal (light) water, but shifted to lower frequencies (see Braun & Smirnov). As a consequence, there is no perceplible absorption in the visible region.
Other Substances
It may well be that other substances which are apparently colourless would show similar faint blue-green colour for the same reason. But only water is encountered in sufficiently large amounts that this colour is highly visible.
Back to the index page “the origins of colour”