Colours of plants and animals
The html formatting and custom instructions have been disabled on this server. The same file with adapted formatting can be found here: https://farbeinf.de/static_html/botzooE.html.
The colours which can be seen on plants and animals can be produced in two quite distinct ways. They may be due to pigments or dyes, or due to special structures which reflect light selectively, mostly in connection with a black pigment which absorbs what is not reflected.
Here a survey of the most common colours shall be given, without diving into details.
In most cases only the names of pigments are mentioned; the corresponding chemical structure may be found in the references given or in Wikipedia; if the name is known, special literature and also suppliers may be found in the internet.
The physical aspects of colour generation have been considered in special sections on dyes,
iridescence and lustre and also on scattering.
Links to original papers and to additional material are given in the text; links to PDF-downloads and secondary literature are numbered and collected at the end of this article.
Plants
Plants produce a variety of colourants; structural colouration is very rare.
The green chlorophyll contained in the leaves enables the plants to use sunlight as energy source for assimilation, synthesizing sugar, starch and cellulose from water and CO2. Chlorophyll can absorb short-wavelength (blue) and long-wavelength (red) light; medium wavelengths are remitted, which makes the leaves green. Green leaves contain other colourful substances in addition, namely carotenoids and xanthophylls, "leaf yellow", in many cases also red anthocyans for UV-protection.
Thus, the colour of the leaves is not for attracting animals, instead, the purpose is photosynthesis.
In contrast, the colours of flowers are produced to bait insects which shall carry the pollen from flower to flower and are rewarded by sweet nectar. Ripe fruits aim at the spreading of their seeds by attracting animals which eat them.
To raise attention, a flower must be different from the leaves.
The easiest way to achieve this is to keep the petals free of colourants, chlorophyll in particular. As the tissue is composed of transparent substances which differ in optical density (index of refraction) and also contains air-filled gaps, there is multiple refraction, reflection and scattering of the light with the effect that most of the incident light is diffusely remitted. The result is white colour like that of lather or snow.
|

White water lily
Nymphaea alba L. |
For gaudy colours, plants synthesize colourants for flowers and fruits. These absorb part of the light, depending on its wavelength. The nonuniform intensity distribution of the remitted light is perceived as colour. Also in this case, multiple refraction, reflection and scattering by the cell structures is responsible for remitting what is not absorbed.

Nasturtium
Tropaeolum majus L.
|

Chicory
Cichorium intybus L. |
There are only few types of flower pigments. The diversity results from the chemical "surroundings" bound to the basic structure of the pigment, the chromophore.
Metal ions (like Fe3+, Al3+) may form complexes with the pigment and change its colour. Im most cases, there is a mixture of several pigments present; single ones are rare.
In the following tables the prevalent pigments of one type are listed together with examples of their occurrence. These are far from being exhaustive. Sources: [1], [2], [3].
Flavonoids
Flavonoids are a major group of plant compounds, comprising numerous, mostly yellow dyes (Latin flavus = yellow).
|
Apigenin | snapdragon, chamomile, yellow dahlias | |
Luteolin | Reseda, dyer's broom, yellow foxglove, dahlia, parsley ... | |
Kaempferol | Buckthorn berries, larkspur, blackthorn berries | |
Quercetin | Marigold, common wallflower, pansies and many flowering plants, apple, pear, apricot, cherry, currants ... | |
Morin | Yellow wood extract from the dyer mulberry | |
Robinetin | Robinia | |
Gossypetin | Cotton, Hibiscus | |
Myricetin | Blackcurrants, potato flowers, Hamamelis
|
|

Dyer's Broom
Genista tinctoria L. |
The interpretation of structural formulas: The main components of organic substances are carbon and hydrogen. The lines represent the chemical covalent bonds, so each line represents a pair of bonding electrons. Double lines represent double bonds. The carbon atom cores themselves are not shown for clarity, at each bend or end of a line there is a carbon atom if there is no symbol of a different element. Hydrogen atoms bound to the carbon atoms are not shown (including the bonding electrons) either. Carbon occurs always tetravalent; if there are less than four lines emerging from a carbon-site, there is a bound hydrogen atom for each missing line. A freely ending line therefore represents a CH3 group.
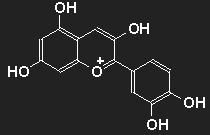
Anthocyanins
The structure of the anthocyanins is very similar to the flavonoids, anthocyanins may be considered to be a subset of flavonoids, but they are blue, violet, purple or red, depending on the "appendages" they have.
They are water-soluble, widespread in flowers, fruits (fruit) and leaves (vegetables). Due to their low resistance to fading not suited as painting or textile dyes; they are used as food additives (colourants). The color depends on the pH value; they can be used as acid-base indicators
[4].
from a chemist's point of view the anthocyanins are glycosides; the sugar – generally a disaccharide – may be separated by dilute acids or enzymatically.
The relationship of the glycosides to their aglycones is expressed often in the name: the former usually end in -in, the latter correspondingly in -idin; e.g. paeonin: paeonidin
[2].
|
Cyanidin | Cornflowers, poppies, red roses, cherries, red cabbage |
|
Delphinidin | Larkspur, monkshood, bluebells, flax, pansies, lavender, sweet peas |
|
Pelargonidin | Pelargoniums, currants, nasturtium, orange dahlias |
|
Paeonidin | Peonies |
|
Petunidin | Petunias |
|
Malvidin | Mallows, hollyhocks, blue grapes |

corn poppy flower
(Papaver rhoeas L.) |

Cornflower
(Centaurea cyanus L.) |
Betalains
Flower and fruit dyes of Centrospermae (comprising the Pink family, Pokeweed family, Purslane- and Amaranth-family), as well as the hat skin of the fly agaric (Amanita muscaria). The betalaines include red-violet betacyans of beetroot (Beta vulgaris var conditiva, belonging to the Chenopodiaceae) and the yellow betaxanthins from cacti.

beetroot from the jar
|
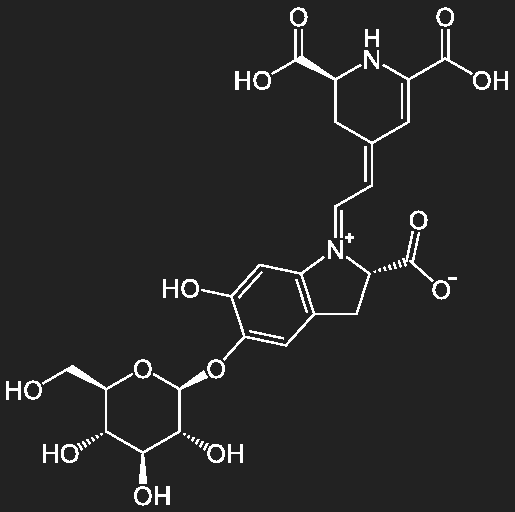
Betanin, beetroot red |

Deptford Pink
Dianthus armeria L. |

Indian Pokeweed
Phytolacca acinosa Roxburgh |
Carotenoids
Name derived from carotene for hydrocarbons (carotenes) and oxygen-containing derivatives (xanthophylls). They are fat-soluble and mostly yellow, orange, red.
|
α-, β-carotene | Carrot, yellow and orange fruits, green leaves | |
Lutein | green leaves (kale, spinach, ...), marigold flowers | |
Capsanthin | Paprika (bell pepper) | |
Capsorubin | Paprika (bell pepper) | |
Canthaxanthin | Chanterelles, crab, flamingo feathers | |
Cryptoxanthin | Oranges | |
Zeaxanthin | Paprika (bell pepper), bladder cherry, green leaves | |
Crocetin | Saffron | |
Lycopene | Tomato, rosehip, calendula
|
|

Bladder cherry
(Physalis alkekengi L.) |
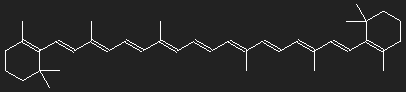
Chemical structure of β-carotene (provitamin A). Carotene contains only carbon and hydrogen
Structural colors
Rare, but they do exist, e.g. in the peacock-fern Selaginella wildenowii from Malaysia which is shimmering blue.
In Kinoshita's book [6] there are some more examples given: Diplazium tomentosum, Lindsaea lucida, Trichomanes elegans, Begonia pavonina, Phyllagathis rotundifolia, Elaeocarpus angustifolius . . . Almost all of them and some more can be seen in the collection of photographs of iridescent plants at Flickr.
Reportedly the berries of Polia condensata from Africa are more intensely coloured than any previously known biological substance (Vignolini et al. 2012),
(Images).
|
_001.jpg)
Peacock-fern Selaginella willdenowii,
Photo: Scott Zona, Source: Wikimedia Commons, license CC BY 2.0
|
The iridescent structures are very similar to those found in beetles, namely stackes of layers with alternating optical density, or helicoidally stacked layers of cellulose microfibrils, see "Beetles".
Animals
In the animal kingdom the ability to synthesize dyes is less developed than in plants. But there are, especially in birds and insects, structural colors that complement the range of pigment colors.
White
The white color of feathers and fur is simply due to the lack of dyes and pigments. Feathers and mammal hair consist of keratin which is colorless transparent. The fine structure of the feathers and tiny air pockets in white hair cause the multiple reflection, refraction, and scattering of light on which the impression of "white" is based.
White insects use special nanostructures for multiple scattering, so that even very thin layers remit almost all incident light, e.g. the wings of the cabbage white (Stavenga et al. 2004). The scanning electron microscope shows numerous elongated "beads" (about 0.5 microns long and 0.15 microns thick) in an irregular arrangement between the longitudinal ribs and the finer transverse ribs of the wing scales. Another example are the scales covering white beetles (Vukusic et al. 2007 [5])
Similar structures are also important for the remission of light when bright colours are produced by pigments.
Melanins
Almost all animals can synthesize melanins. The furs of mammals, feathers of birds, the exoskeletons of many insects and the black areas on butterfly wings are colored by melanin.
Our body produces melanin too: tan of the skin and hair colour are due to it.
One distinguishes between eumelanin, dark brown to black, and pheomelanin, yielding yellowish, reddish to reddish brown. Their concentration and mixing ratio determines hair colour and complexion [7].
Among the insects, the butterflies are conspicuous due to their colors. Black, brown, red, orange, and yellow can be produced by dyes or pigments in the scales on the wings. Blue and green on butterfly wings are almost always structural colours that come about without any stain.
The following information about colours of butterfly wings comes largely from the article "Coloration: Patterns and Morphogenesis" by H. Frederik Nijhout [8]. There are also data on the chemistry of pigments mentioned here only by name.
Pterins
The pigments in the wings of Pieridae (Whites, Yellows) have been analyzed in the late 19th Century
(F. Gowland Hopkins, 1895) [9].
 |
 |
 |
Large White
Pieris brassicae ♀ | Brimstone
Gonepteryx rhamni ♂ | Orange-tip
Anthocharis cardamines ♂ |
Gowland Hopkins found that the dark colors (black, brown) are caused by pigments strongly bound to the chitin of the wing scales, while the bright ones, i.e. white, yellow, orange, are like a powder like substance on the chitin of the scales and could be released with hot water from the wings. From the chemical analysis, he concluded that the white pigment is nothing else than uric acid, while the yellow to orange pigments are derivatives of uric acid.
Later it turned out that it was not uric acid and its derivatives which Gowland Hopkins had isolated and studied, but new, then still unknown compounds that are known today under the name of pterins and pteridine derivatives. (The names are derived from the first record in butterfly wings, Greek pteron = wing.) Leukopterin is the white, xanthopterin the yellow pigment (Pfeiler 1970 [10]).
Leukopterin incidentally absorbes in the ultraviolet (Morehouse et al. 2007). For us, the cabbage whites are white, but birds, bees and cabbage whites see things differently and see the difference from a white flower that reflects UV too.
Papiliochromes
A group of pale yellow pigments found in the Papilionidae, whose best known member in Europe is the swallowtail.
|

Swallowtail
Papilio machaon |
Ommatins
Brown, Red, Orange, Yellow, Buff are caused by pigments from the group of ommatins (ommochromes, "eye colors" of insects). Xanthommatin (yellow, brown), rhodommatin (red).
 |
 |
 |
Red Admiral
Vanessa atalanta |
Small Tortoiseshell
Aglais urticae | Meadow Brown
Maniola jurtina ♀ |
Flavonoids
Flavonoids are plant pigments that can not be synthesized by Lepidoptera, but are consumed in the diet of the caterpillar. In almost all families of butterflies (Papilionoidea) white and yellow flavonoids have been detected.
Carotenoids
|
The carotenoids of animals are usually conversion products of plant food carotenoids. They are as responsible for the yellow to red color of many bird feathers (such as flamingos) and of ladybugs, Colorado beetles and many other insects (Britton et al. 1977)., and also for the red of many crustaceans [1].
However, carotenoids have not yet been detected in butterflies' wings. | |

seven-spot ladybird
Coccinella septempunctata |

22-spot ladybird
Psyllobora 22-punctata | |
Bile pigments
|
Dyes resulting from the degradation of hemoglobin have first been found in bile (bilirubin, biliverdin, Latin bilis = bile). Chemically related dyes are therefore called bile pigments, even if they have no connection with bile.
The rare green dyes from butterfly wings (Geometridae, Sphingidae, Papilionidae) are attributed to the bile pigments [8].
Sky-blue color from a bile pigment was found in the neotropical genus Nessaea (Nymphalidae) [11], according to [8].
|

Southern Grass Emerald
Chlorissa cloraria | |
Green
Supposedly green coloration of many insects is due to chlorophyll taken from plant food. This can be read again and again, but if it's more than a guess, and for what kind of insects it is true, I do not know. Original papers confirming that, I have not found yet. In contrast, Saito and Shimoda (1997) [12] write: Caterpillars are often green in body color [...] A green coloration has also been observed in the hemolymph and integument of various insects. The green color results from a combination of a blue pigment, biliverdin, and yellow pigments, carotenoids.
From caterpillars of the Great Cabbage White, Pieris brassicae, the blue pigment pterobilin was isolated (W. Rüdiger et al. 1968). This substance was first detected in the wings of P. brassicae (Wieland & Tartter 1940). ("Bei der Verarbeitung der Flügelpigmente von rund 1 Million Kohlweißlingen [...] konnte der interessante Farbstoff in einer Menge von 200 mg isoliert und näher charakterisiert werden.")
("During the processing of the wing pigments of some 1 million cabbage whites [...] the interesting pigment could be isolated in an amount of 200 mg and characterized.") It belongs to the family of bile pigments and probably causes the greenish color of brimstone-females (my guess).
In the larvae of the tobacco hornworm Manduca sexta, insecticyanin, a blue dye (a biliprotein) has been found which is present together with a yellow carotenoid and causes the green colour (Kawooya et al. 1985),
and also in caterpillars of the Convolvulus Hawk-moth Agrius convolvuli [12]. Blue biliproteins have been detected in caterpillars of Rhodinia fugax (from the family Saturniidae – peacock moths), which are light green on the upper, dark green at the lower side (Hitoshi Saito 2001).
In cases where it has been examined, the green is therefore not due to chlorophyll.

Snout caterpillar
Hypena proboscidalis |

Sawfly larva |

Thistle tortoise beetle
Cassida rubiginosa
|

Great Green Bush-Cricket
Tettigonia viridissima |

Green lacewing
Chrysopinae sp.
|

Green shield bug
Palomena prasina |
In the lacewing Chrysopa carnea, biliverdin (and xanthommatin) has been detected
(Rüdiger et al. 1970), biliverdin in the migratory locust Locusta migratoria and the praying mantis Mantis religiosa (M. Passama-Vuillaume). In view of the similar structures of hemoglobin and chlorophyll, it seems plausible that biliverdin and other bile pigments are formed by decomposition of chlorophyll.
Structural colors
The green color of butterflies and beetles is in most cases, the blue is almost always produced without coloured dyes or pigments: special structures of the scales on butterfly wings ensure that certain wavelengths of light are strongly reflected, others pass through and are absorbed by dark pigment. In beetles the cause of selective remission is in most cases a stack of transparent layers with alternating large and small refractive index.
|
|

Green Hairstreak
Callophrys rubi
|

Holly Blue
Celastrina argiolus |

Scarce Copper
Lycaena virgaureae
|
|
|

Green Forester
Adscita statices |

Common Cuckoo Wasp
Chrysis ignita |

Common Greenbottle
Lucilia caesar
|

Leaf beetle
Cryptocephalus sericeus
|

Flower chafer
Protaetia cuprea
|

Leaf beetle
Chrysolina fastuosa |

Green Nettle Weevil
Phyllobius urticae |

Blue Shieldbug
Zicrona caerulea
|

Deer Fly
Chrysops relictus |
Besides the insects, many birds show structural colours in their plumage. The peacock is the best known example.
 |
 |  |
Mallard drake
Anas platyrhynchos | Peacock's feather seen
under different angles |
Hummingbirds are famous for their vivid iridescent colours.
Structural colors are also found in inorganic materials. The simplest case is that of thin transparent layers, as seen on soap bubbles or oil films on wet asphalt. Many layers with varying index of refraction make the labradorite (a feldspar mineral) iridescent with blue, greenish yellow or other colours, and the reason for the glinting colours of precious opal is its composition of submicroscopic, almost equal silica beads arranged regularly over large domains.
The structures which produce these colours are dealt with in a separate section .
Another blue – and green
Blue pigments are rare in the animal kingdom, especially in vertebrates. Instead of pigments, structural colours occur, but there is another possibility to produce blue, namely the exploitation (or imitation) and optimization of the Tyndall effect. Particles which are much smaller than the wavelengths of visible light, scatter short-wavelength light much more strongly than long-wave. If they are distributed randomly – the Tyndall effect – the scattered light is then sky-blue and before a dark background which absorbs what is not scattered, the blue colour is at its best.
The blue of feathers, if they do not shimmer, comes about like that as well as the blue colour on the face and buttocks of the male mandrill, and the colour of the iris of blue eyes.
Many dragonflies and damselflies show a non-iridescent sky-blue on their body. Reptiles and amphibians generate their green with additional yellow pigments (carotenoids) as a yellow filter.
However, recent studies have shown that the scattering is not incoherent, as in the classic Tyndall effect (Prum et al., 1999a, 1999b, 2004a, 2004b).
The scattering centers are not distributed completely randomly. This enhances the blue colour, see the discussion given here.

Common Blue Damselfly
Enallagma cyathigerum |

European tree frog, Hyla arborea
(Photo: Ineptus) |

Mandrill, Mandrillus sphinx
Photo: Robert Paul Young
Source: Wikipedia, license CC BY 2.0
|
This method to produce blue has not yet been found on lepidopteran wings. It was supposed that the tropical blue swallowtail Papilio zalmoxis makes use of it, but this has been refuted (Prum et al. 2006). Instead a blue pigment has been detected, but its chemical structure has not yet been analyzed.
Back to the overview, to the section on dyes, on Structural colors or on scattering.
Sources and links
|
[1] | Wikipedia: Flavonoid, Anthocyanin, Betalain, Carotenoid, etc., etc.
|
|
[2] | Anonymous: document_469.pdf (www.ichemlab.at)
(PDF-download), in German. |
|
[3] | Ingo Klöckl: farbchemie.html |
|
[4] | Professor Blume's Tip # 111 (in German) |
|
[5] |
Pete Vukusic,
Benny Hallam,
Joe Noyes:
Brilliant Whiteness in Ultrathin Beetle Scales. Science 19 January 2007:
Vol. 315 no. 5810 p. 348
DOI: 10.1126/science.1134666 (PDF-download)
SOM (PDF) (Supporting Online Material, Figs. S1–S4)
|
|
[6] | Shuichi Kinoshita: Structural Colors in the Realm of Nature. World Scientific 2008, ISBN-13 978-981-270-783-3
|
|
[7] | Margareta Wallin: Nature's palette – How animals, including humans, produce colours: bioscience explained Vol 1 No 2
(PDF-download) |
|
[8] | H. Frederik Nijhout: "Coloration: Patterns and Morphogenesis"
im
Handbuch der Zoologie /Handbook of Zoology,
herausgegeben von Niels P. Kristensen; Verlag Walter de Gruyter & Co.,
Volume IV, Band 2 (2003): Lepidoptera, Moths and Butterflies: Morphology, Physiology, and ..., S. 24 ff.
|
|
[9] | F. Gowland Hopkins, 1895:
The Pigments of the Pieridae: A Contribution to the Study of
Excretory Substances Which Function in Ornament.
Phil. Trans. R. Soc. Lond. B 1895 186, 661-682
doi: 10.1098/rstb.1895.0015 (PDF-download) |
|
[10] |
Edward J. Pfeiler, Jr.:
The Effect of Pterin Pigments on the Wing Coloration of Four Species of Pieridae (Lepidoptera), Journal of Research on the Lepidoptera 7(4): 183–189. 1968(1970)
(PDF-download).
|
|
[11] |
Richard Irwin Vane-Wright: The colouration, identification and phylogeny of Nessaea butterflies (Lepidoptera: Nymphalidae) Bulletins of the British Museum (Natural History): Entomology series – vol.38 (1979) no.2.
|
|
[12] | Hitoshi Saito and Masami Shimoda: Insecticyanin of Agrius convolvuli: Purification and Characterization of the Biliverdin-Binding Protein from the Larval Hemolymph. Zoological Science 14: 777–783 (1977)
(PDF-download) |
Legal Information Data Privacy












_001.jpg)

































